STOP BLOQ -
Surveillance and Treatment to Prevent Fetal Atrioventricular Block Likely to Occur Quickly
How does the heart beat?
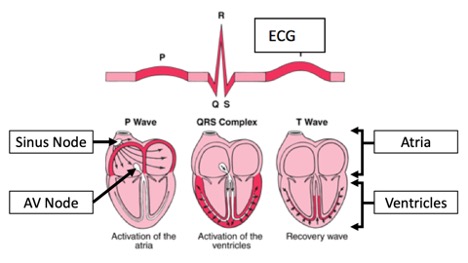
- The heartbeat starts in the sinus node which is in the upper portion of the right atrium (Figure 1).
- The electrical impulses from the sinus node sweep down through the right and left atria (atrial activation) and are represented by the p-wave on the electrocardiogram (ECG). Atrial contraction is silent, that is it cannot be heard by the stethoscope or a Doppler monitor.
- As the atria contract, the electrical impulses reach the atrioventricular (AV) node (seen as the PR interval on the ECG), and then are relayed to the lower chambers of the heart, the ventricles (ventricular activation). This is represented by the QRS complex on the ECG.
- When the ventricles contract, blood is pushed out to the arteries. This is the “heartbeat” that is heard by the stethoscope and the Doppler monitor.
- The heart then recovers (T-wave) and gets ready for the next beat.
What is atrioventricular block?
- Atrioventricular block (AVB) occurs when inflammation and/or scarring (fibrosis) of the AV node prevents electric signals from the atria from reaching the ventricles (1, 2).
- Almost all AVB develops in the second trimester of pregnancy (18-26 weeks of gestation).
- Fetal AVB occurs in 2% of pregnant women who carry anti-Ro/SSA antibodies (3, 4, 5).
- Fetal AVB secondary to anti-Ro/SSA antibodies is part of the spectrum called “neonatal lupus” but it does not mean the baby has lupus.
What are Anti-Ro/SSA Antibodies?
- These are autoantibodies which react with a protein in side all cells referred to either as Ro or SSA. Sometimes you will see the term anti-Ro or anti-SSA or sometimes anti-Ro/SSA.
- There are two proteins which have been referred to as Ro which are Ro60 and Ro52. Antibodies to both or either of these proteins are associated with fetal AVB.
- We therefore will evaluate both anti-Ro52 and anti-Ro60 antibody levels in Step 1 of STOP BLOQ.
- Some individuals with anti-Ro/SSA antibodies have autoimmune diseases such as lupus or Sjogren’s syndrome, but many have no symptoms at all.
Progression of AVB
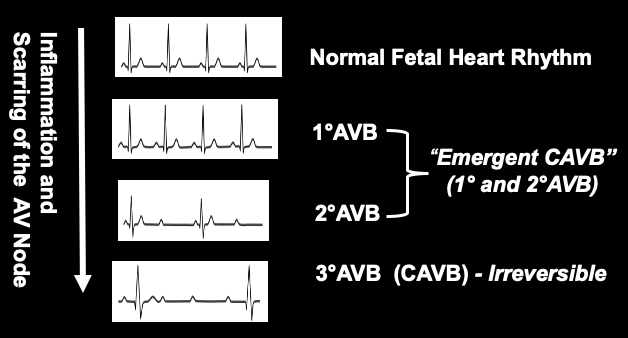
- There are 3 types of AVB which vary based on the damage to the AV node.
- The fetal heart rate and rhythm depend on the degree of damage to the AV node:
- Early damage (1° AV block): the electric signals from the atria are delayed but reach the ventricles. The fetal heart rate and rhythm are normal.
- Evolving damage (2° AV block): Some of the electric signals from the atria do not reach the ventricles, but others do. The fetal heart rhythm is irregular.
- Complete damage (3°AV block): No electric signals from the atria reach the ventricles. The fetal heart rate is slow (≤70 beats per minute) and the rhythm is regular.
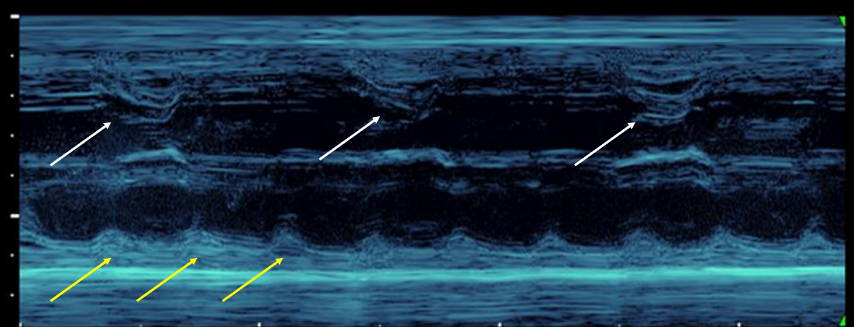
- The M-Mode image below (Figure 2) shows a ventricular rate (top tracing) of 50 beats per minute (white arrows) and an atrial rate of 145 bpm (yellow arrows). Note there is no relationship between the atria and the ventricles. Normally there is a one-to-one relationship.
- Thus, there is a progression from normal fetal heart rhythm (NR) to 3°AVB, also referred to as “Complete AVB”, or CAVB (Figure 3).
- 3° AVB is irreversible, and is associated with an 18% perinatal mortality rate (6).
- This exceeds the mortality rate for all non-cardiac congenital anomalies combined.
- Almost all survivors of 3° AVB require the lifelong use of a pacemaker, with its associated complications (7, 8).
How is AVB diagnosed?
- Fetal heart rate monitoring (FHRM), using a commercial Doppler monitor, is an effective early detection method for AVB.
- When an irregular heart rhythm is detected, an echocardiogram (or echo) is used to diagnose AVB.
These two videos show echoes of a healthy fetal heart (left) and a heart with AVB (right)
"Likely to Occur Quickly"
- The emergent CAVB period (including 1° AVB and 2° AVB) may be the only window of opportunity for anti-inflammatory treatment to restore NR (9).
- Progression from normal rhythm to CAVB (3° AVB) can occur in less than 24 hours (10).
- Weekly or biweekly fetal echos may therefore be insufficient to detect emergent CAVB and prevent the development of CAVB (11).
Fetal Heart Rate Monitoring
- Our team has shown that frequent, at-home fetal heart rate monitoring (FHRM) is an easy and accurate method for detecting irregular heart rhythms associated with emergent CAVB (12).
- In combined results from studies of 275 anti-Ro/SSA pregnancies, mothers consistently distinguished between normal and abnormal fetal heart rhythms and successfully contacted their providers with abnormal results (13).
- Furthermore, FHRM was found to be feasible and empowering to the mothers in these studies.

Why STOP BLOQ?
- Surveillance and Treatment tO Prevent Fetal AV Block Likely to Occur Quickly
- Combines the expertise of Fetal Cardiologist Bettina F. Cuneo, MD (University of Arizona College of Medicine) and Rheumatologist Jill P. Buyon, MD (NYU School of Medicine)
- Over thirty participating sites across the US and Canada
- A prospective observational trial with 3 steps:
- Screening for high titer anti-Ro60 or Ro52
- Surveillance by FHRM 3X daily and weekly or biweekly echo
- Treatment of 2° AVB identified by FHRM and confirmed by echo
Impact
STOP BLOQ presents a unique opportunity to reverse the inflammatory/fibrotic effects of Ro52/Ro60 antibodies to the fetus, and thereby prevent lifelong disability. This study is grounded in:
- Strong preliminary data,
- Interdisciplinary collaboration
- National expertise
It is anticipated that this study will:
- Decrease the incidence of fetal 3° AVB
- Yield evidence-based management guidelines
- Set a precedent for universal pre-natal screening for anti-Ro
- Reduce costlier echo surveillance (and consequently the number of clinic visits)
- Empower mothers in their own health care
References
- Llanos C, Friedman DM, Saxena A, Izmirly PM, Tseng CE, Dische R, Abellar RG, Halushka M, Clancy RM, Buyon JP. Anatomical and pathological findings in hearts from fetuses and infants with cardiac manifestations of neonatal lupus. Rheumatology (Oxford). 2012;51(6):1086-92
- Clancy RM, Kapur RP, Molad Y, Askanase AD, Buyon JP. Immunohistologic evidence supports apoptosis, IgG deposition, and novel macrophage/fibroblast crosstalk in the pathologic cascade leading to congenital heart block. Arthritis Rheum. 2004;50(1):173-82
- Levesque K, Morel N, Maltret A, Baron G, Masseau A, Orquevaux P, Piette JC, Barriere F, Le Bidois J, Fermont L, Fain O, Theulin A, Sassolas F, Pezard P, Amoura Z, Guettrot-Imbert G, Le Mercier D, Georgin-Lavialle S, Deligny C, Hachulla E, Mouthon L, Ravaud P, Villain E, Bonnet D, CostedoatChalumeau N, Lupus neonatal g, Group of c. Description of 214 cases of autoimmune congenital heart block: Results of the French neonatal lupus syndrome. Autoimmun Rev. 2015;14(12):1154-60
- Buyon JP, Clancy RM, Friedman DM. Cardiac manifestations of neonatal lupus erythematosus: guidelines to management, integrating clues from the bench and bedside. Nat Clin Pract Rheumatol. 2009;5(3):139-48
- Cimaz R, Spence DL, Hornberger L, Silverman ED. Incidence and spectrum of neonatal lupus erythematosus: a prospective study of infants born to mothers with anti-Ro autoantibodies. The Journal of Pediatrics. 2003;142(6):678-83
- Izmirly PM, Saxena A, Kim MY, Wang D, Sahl SK, Llanos C, Friedman D, Buyon JP. Maternal and fetal factors associated with mortality and morbidity in a multi-racial/ethnic registry of anti-SSA/Roassociated cardiac neonatal lupus. Circulation. 2011;124(18):1927-35.
- Wilhelm BJ, Thone M, El-Scheich T, Livert D, Angelico R, Osswald B. Complications and Risk Assessment of 25 Years in Pediatric Pacing. Ann Thorac Surg. 2015;100(1):147-53.
- Cheng P, Gutierrez-Colina AM, Loiselle KA, Strieper M, Frias P, Gooden K, Blount RL. Health related quality of life and social support in pediatric patients with pacemakers. J Clin Psychol Med Settings. 2014;21(1):92-102
- Cuneo BF, Ambrose SE, Tworetzky W. Detection and successful treatment of emergent anti-SSA mediated fetal atrioventricular block. Am J Obstet Gynecol. 2016;215(4):527-8.
- Cuneo BF, Sonesson SE, Levasseur S, Moon-Grady AJ, Krishnan A, Donofrio MT, Raboisson MJ, Hornberger LK, Van Eerden P, Sinkovskaya E, Abuhamad A, Arya B, Szwast A, Gardiner H, Jacobs K, Freire G, Howley L, Lam A, Kaizer AM, Benson DW, Jaeggi E. Home Monitoring for Fetal Heart Rhythm During Anti-Ro Pregnancies. J Am Coll Cardiol. 2018;72(16):1940-51
- Friedman DM, Kim MY, Copel JA, Davis C, Phoon CK, Glickstein JS, Buyon JP. Utility of cardiac monitoring in fetuses at risk for congenital heart block: the PR Interval and Dexamethasone Evaluation (PRIDE) prospective study. Circulation. 2008;117(4):485-93
- Cuneo BF, Moon-Grady AJ, Sonesson SE, Levasseur S, Hornberger L, Donofrio MT, Krishnan A, Szwast A, Howley L, Benson DW, Jaeggi E. Heart sounds at home: feasibility of an ambulatory fetal heart rhythm surveillance program for anti-SSA-positive pregnancies. J Perinatol. 2017;37(3):226-30.
- Cuneo BF, Sonesson SE, Levasseur S, Moon-Grady AJ, Krishnan A, Donofrio MT, Raboisson MJ, Hornberger LK, Van Eerden P, Sinkovskaya E, Abuhamad A, Arya B, Szwast A, Gardiner H, Jacobs K, Freire G, Howley L, Lam A, Kaizer AM, Benson DW, Jaeggi E. Home Monitoring for Fetal Heart Rhythm During Anti-Ro Pregnancies. J Am Coll Cardiol. 2018;72(16):1940-51
